
There are five electron pair geometries: linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. In fact for NO 2 –, a bent molecular geometry, the bond angle is 115.4 o.ĭiscussed below are the electron pair and molecular geometries. When one of the bonds become a lone pair, the molecular geometry is bent and the bond angle is less than 120 o.
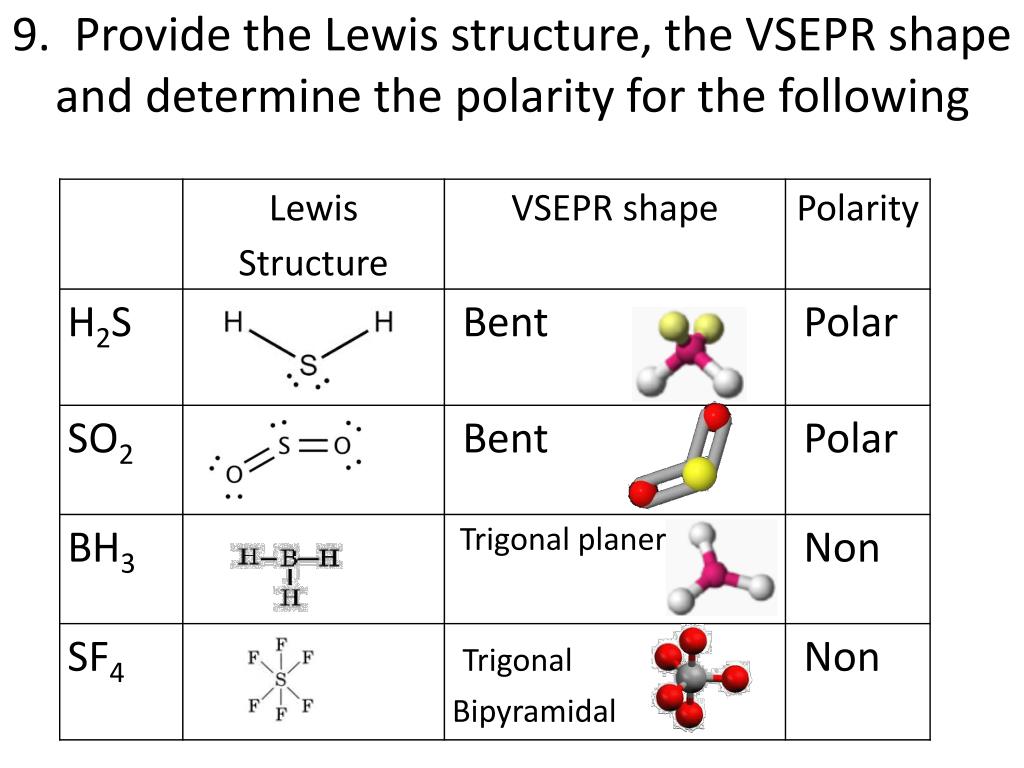
For example, for a trigonal planar molecular geometry, like BF 3, the bond angles are 120 o. When lone pairs are involved the bond angles in the molecule become compressed. This is because the lone pair of electrons is only under the influence of one nucleus and have more room to spread out whereas bonding electrons are held more tightly between two nuclei. If there are one or more lone pairs (or single electron), the molecular geometry is different. If there are no lone pairs, the molecular geometry is the same as the electron pair geometry. Then count the charge clouds to determine electron pair geometry. To predict electron pair geometry and molecular geometry, first draw the Lewis structure. You will need to know the electron pair geometries and molecular geometries. The table below gives the electron pair geometries and molecular geometries for different molecules and ions. It is only when there are one or more lone pairs on the central atom that molecular geometry differs from the electron pair geometry. If the central atom does not have any lone pairs (or a single electron), the molecular geometry is the same as the electron pair geometry. The molecular geometry only takes into consideration the number of bonds. The electron pair geometry is determined by the total number of charge clouds around an atom. Looking at the water molecule, we see the oxygen has 4 electron groups (charge clouds) surrounding it, two H-O bonds and two lone pairs of electrons. These electron groups are referred to as charge clouds. A single bond, double bond, triple bond, a lone pair, or a single electron count as a single electron group. Here we will discuss both electron pair geometries (also called electron group or electron domain geometries) and molecular geometries (also called molecular shapes).Ī group of electrons is any number of electrons that occupies a localized region around an atom. We use the Valence Shell Electron Pair Repulsion (VSEPR) model to predict the shapes of molecules. The regions of electrons density, bonds and nonbonding electrons, will repel one another and therefore orient as far away from each other as they possibly can to minimize these repulsions.
If four balloons are tied together, each balloon will orient to a corner of a tetrahedron with angles of 109.5 o.Įlectrons in molecules will act similarly to the balloons. If we add a third balloon, we see the balloons point to the corners of an equilateral triangle with angles of 120 o apart. In fact, the balloons are 180 o apart and have a linear orientation.

The balloons will point away from one another as shown in the figure below. Imagine two equal sized balloons that are tied together.
#VSEPR SHAPES OF BENT HOW TO#
Molecules and ions are three dimensional and we have only learned how to draw two dimensional shapes.
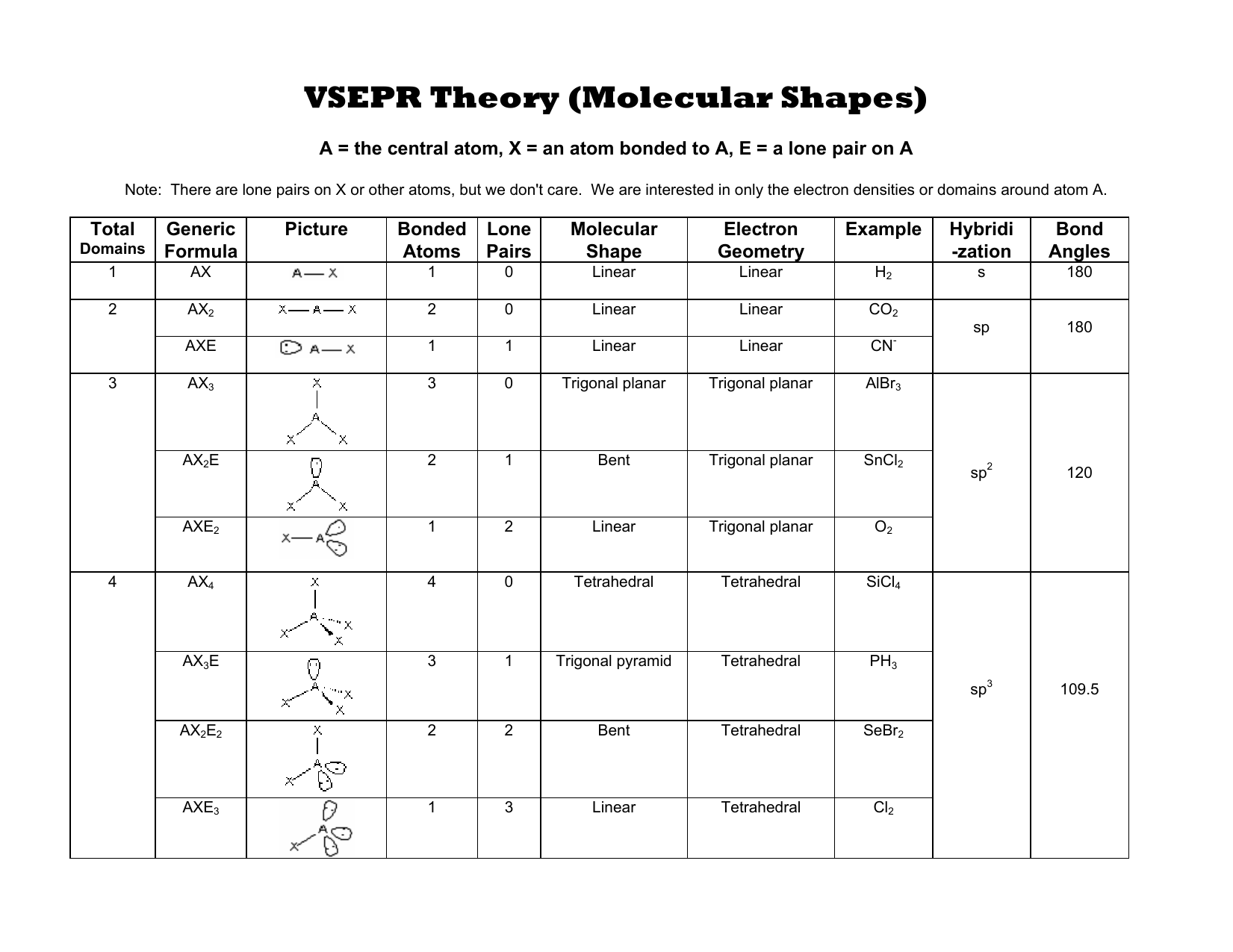
Lewis structures do not tell us anything about molecular shape. Lewis structures were used previously to determine how the atoms are connected in a molecule and show where the lone pairs of electrons are located.


 0 kommentar(er)
0 kommentar(er)
